


I had a chance to spend some range time
with a Taurus
model 627SS Tracker chambered for .357
Magnum / .38 Special, 4" barrel, shooting Prvi Partizan
(PPU) 158 gr (10.2 gram) Lead Round Nose (LRN) 38 Special
bullets. I previously posted a review of
the Taurus 627SS and loader accessories. On this trip, I was
interested in how the gun would perform with lead bullets and wanted to
know what failure mode would show up first. I want to be
confident in self-defense situations, but also might trust this gun in
the field against larger wild animals where it's not kept in perfect
condition.
I shot about 300 rounds over 3 days and
the only cleaning I did was to wipe down the front sight with alcohol
or water to remove the smokey smudge that builds up after about 80
rounds. Alcohol pads are great to remove the soot
collection.
Licking your finger to wipe soot away works less well, and probably
introduces lead into your body. Half way
through the third day, instead of shooting with a free hand double
action accuracy of about 5 minutes of arc (MOA), the bullets started
dropping about 8 cm over 15 yards. Precision was still holding,
but all the bullets were curving low about 8 cm on target.
Adjusting the sights
didn't change the impact point, and that made me really suspicious
because the only way that could happen is if the bullets were being
lobbed out of the barrel at a very slow speed. If you do a gravity calculation, you can
see that a bullet falls about 8 cm in 0.128 seconds. Because I
was 15 yards (45 feet) away creating the 8 cm drop, that means the
bullet was traveling 45 feet in 0.128 seconds or 350 fps. They're
specified by the manufacturer at 890 fps, but they were coming out of
the barrel at 350 fps - a 60% loss in velocity! That means the
barrel was really clogged, and I'm lucky it was a barrel meant to
handle 357 pressures while I was shooting only 38 specials!
The first two photos show what I found back home, looking down the front of the barrel, back illuminated with a LED illuminator. Looks like pictures of cholesterol plugged arteries! The lead was pretty much untouched after running a brash brush about 50 strokes and trying three different bore cleaners. Looking at the cleaners I used, they all indicated they would remove copper. Yup, I guess so. But not lead!
The third photo is the finished barrel after about three hours of work including dissolving lead into liquid mercury and hundreds of strokes with pure copper abrasive. If you can see two scratches, it's because I first tried to (and failed to) poke the lead chunks out with a screwdriver. Resolution/focus of the pictures is the best I can do without a macro lens on my camera.
| BEFORE |
BEFORE | AFTER |
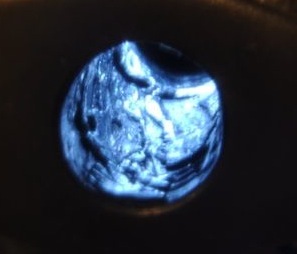 |
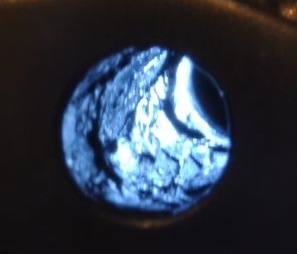 |
 |
Below are the tools I used. Towel covers the work surface.
Red flashlight with LED on the end of a flexible shaft illuminates the bore interior. Chore
Boy dish cleaning abrasives ($1.21 after tax) were unfolded (they're
normally folded in
on themselves to keep them a ball), magnifying glass, dental spoon,
biological disection poker, chemistry spatula, bore cleaning rod with
copper filaments pushed through the end loop.

Photo below shows what about 75 strokes
of copper strands accomplished. Clearly a pile of lead started to
accumulate on the towel. This would work, but would take a long
time. This is similar to the Lewis
Lead Remover toolkit that uses brass weaved disks instead of copper filaments. Others recommend wrapping the copper
filaments strands around a normal brash brush.

You can read several places on the web
about using hydrogen peroxide and vinegar to form a chemical complex
with the lead and create Pb2(CH3COO)2
(lead (II) acetate), possibly lead (IV) acetate (need to research this
a bit more). Most of the lead will dissolve into the liquid
solution you but into the barrel and the rest can be wiped off the
barrel with a cleaning patch after you remove the liquid. Lead
acetate is water soluble, so this can gets into your system
easily and even through your skin. Realize that because there is
probably already lead residue on your hands, just getting the
peroxide/vinegar solution onto your hands will do the same reaction and
create the lead acetate solution right on your skin. Although it used to be a medicinal
drug, we now know lead acetate
is toxic.
If clean your barrel with this method, you'll have toxic solution coming out and toxic crud on your barrel
wipes. If you there is a company using chemicals or a college nearby, ask to speak with
their lab manager and ask if you can add your liquid to their liquid
inorganic waste collection. They probably have a waste
stream for the used cleaning patches, too.
You can lower some of the threat of
lead acetate
solution by putting it into a strong liquid acid to push the hydrogen
back on
the acetate and make acetic
acid (vinegar or film camera fixing agent) and a lead precipitate. The toxic problem
is
the lead part of the equation. The lead turns into lead chloride
if you use hydrochloric acid or lead sulfate
if you use sulfuric acid (battery acid). Both of these new lead
compounds are also toxic (see MSDS lead
sulfate, lead chloride).
However, unlike lead acetate, they are insoluble in water, so they
precipitate out of
water so you can filter it and dispose of it easier than the lead
acetate. These precipitation reactions are some of the
"qualitative analysis" chemical reactions freshman college students
learn.
For perspective, lead sulfate is the white powder that forms on your car
battery lead terminals,
so you have probably dealt with it before, blown it away with an air
compressor hose, or washed it away with the hose down the
driveway. No, you
should not do this. You'll probably get
Parkinson's disease if you continue being careless. Notice that car
mechanics are now do work with latex gloves, unlike the old day.
I have some liquid
mercury I've been trying to get rid of or find a use for, so I decided
to try that method. BTW, if you want to try this, I have lots of
extra mercury and I'd be happy to sell you small quantities. If
you have a clear lab bench to work and understand how to work around
chemicals, it can be a safe, quick, and thoroughly removing lead.
Federal law requires only 1 pound maximum can be shipped between
private parties, and it needs to be done via FedEx - USPS and UPS won't
do it.
Notice in the picture below, the mercury
is shiny and has a high surface tension (tends to puddle up easily and
won't wet the container it's in). After reacting with the lead it
will be different. I have the tip of a little suction bulb
showing on the right side of the picture, but don't plan to move
mercury that way. It doesn't pick up well because it's so
heavy. That is one of the reasons mercury protocol is to always
have a non-metallic catch basin under your work so that you catch any
spills and can lift the mercury back up and pour it into a storage
container.

As shown below, I used electrician's tape to plug the front and side vents. Next time I would try to find something that the mercury/lead didn't stick to. (Next time I may experiment with foam ear plugs. I'm not sure if that would block the mercury or allow it to get into all the little foam holes.) After sealing off the front of the gun barrel, I poured the mercury from the film canister into the gun barrel. Lastly, I sealed the chamber with another piece of tape. Shake. Tilt around. Tip back and forth. Agitate. Basically move the mercury around to react with the lead in the barrel.







